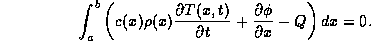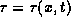 be the thermal density which is the amount of
thermal energy per unit volume.
The Thermal energy density may vary from one cross section to another.
It may also change with respect to time.
be the thermal density which is the amount of
thermal energy per unit volume.
The Thermal energy density may vary from one cross section to another.
It may also change with respect to time.
Consider a rod oriented horizontally. The rod is assumed to have constant cross-sectional area A. Further the material properties of a cross section of the rod are assumed to be uniform. The sides of the rod are assumed to be perfectly insulated so that no thermal energy can pass through the surface.
The left end of the rod is at x=0 and the right end is at x=L, the
length of the rod.
Let 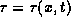 be the thermal density which is the amount of
thermal energy per unit volume.
The Thermal energy density may vary from one cross section to another.
It may also change with respect to time.
be the thermal density which is the amount of
thermal energy per unit volume.
The Thermal energy density may vary from one cross section to another.
It may also change with respect to time.
The heat energy of a given section of the rod is the volume of the
section times the thermal density.
For a thin slice of the rod contained between x and 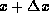 ,
the heat energy is given by
,
the heat energy is given by
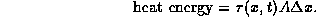
Consider any finite segment of the rod from x = a to x = b.
Hence, the total heat energy is 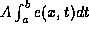 .
The rate of change of the heat energy flowing across the rod per unit
volume per unit time is
.
The rate of change of the heat energy flowing across the rod per unit
volume per unit time is
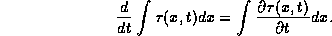
The heat energy of a section of the rod changes in time only due to
heat energy flowing across the edges of the section of the rod.
The energy flowing across the section's boundaries per unit time is
the heat flux 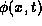 .
If
.
If 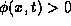 heat is flowing to the right.
For a thin slice of the rod contained between x and
heat is flowing to the right.
For a thin slice of the rod contained between x and 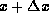 ,
the heat energy flowing across the boundaries of the slice is
,
the heat energy flowing across the boundaries of the slice is
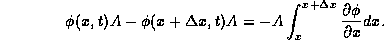
for any finite segment of the rod from x = a to x = b, heat energy flowing across the boundaries per unit area is
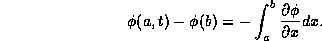
Internal heat sources of thermal energy are given in terms of
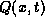 the heat energy per unit volume generated per unit time.
The conservation of heat energy states that the rate of change of the
heat energy in time is equal to the heat energy flowing across the
boundaries per unit time plus the heat energy generated in the section
per unit time.
Hence,
the heat energy per unit volume generated per unit time.
The conservation of heat energy states that the rate of change of the
heat energy in time is equal to the heat energy flowing across the
boundaries per unit time plus the heat energy generated in the section
per unit time.
Hence,
This integral must be zero for every a and b.
A more familiar form, but less general, is arrived at from the fact that for this integral to be zero for every choice of a and b, the integrand must be identically zero. Hence it can be written as
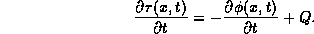
The heat capacity of a substance is the heat energy that must be supplied
to a unit mass of substance to raise its temperature one unit.
Similarly, the thermal energy can be defined as the energy that takes
to raise the temperature from a reference 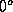 to its actual temperature
to its actual temperature
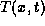 .
Hence the thermal energy and temperature satisfy the following equation.
.
Hence the thermal energy and temperature satisfy the following equation.
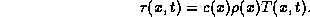
Therefore,