


Next: Bingham's correlation Lab
Up: Exercises
Previous: Exercises
Nernst equation describes the relationship between
the cell potential and the concentration of the cell components.
The cell potential 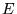 is given by:
is given by:
Where 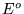 is the cell potential at standard conditions,
is the cell potential at standard conditions,
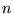 is the number of moles of electrons,
is the number of moles of electrons,
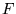 is the charge per mole of electrons,
is the charge per mole of electrons,
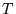 is the
temperature, R is the gas constant,
and
is the
temperature, R is the gas constant,
and 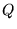 is the reaction quotient.
is the reaction quotient.
Write a main() program that receives as input the
cell potential at standard conditions,
the number of moles of electrons,
the charge per mole of electrons,
the temperature, and the reaction quotient; and
and outputs the
cell potential.
The input is from the standard input, the keyboard. The results
are sent to the standard output, the screen.
J. C. Diaz
2004-01-19